White Paper Measuring Monochloramine without reagents
- Home
- White Paper Measuring Monochloramine without reagents
Monochloramine Analyzers for Drinking Water Treatment
Introduction
Monochloramine is a common disinfectant used in drinking water treatment. Because monochloramine is more stable and less reactive than free chlorine, it allows for disinfection without the potential formation of harmful by-products such as trihalomethanes. However, the efficacy of monochloramine, and the various advantages it brings, depends on the ability to accurately measure it in the water treatment process. In this paper, we will discuss the benefits of using online monochloramine analyzers for the monitoring of monochloramine in the drinking water treatment process.
Background
Why Chloraminate
Monochloramine is used as a drinking water disinfectant for several key reasons
Effectiveness as a Secondary Disinfectant
Monochloramine provides longer-lasting disinfection compared to chlorine as water moves through pipes to consumers[2]. While it is a weaker primary disinfectant than chlorine, monochloramine is more stable and remains effective over longer distances in the water distribution system[5]. This makes it particularly useful for secondary disinfection to maintain water quality throughout the distribution network.
Reduced Disinfection Byproducts
One of the main advantages of using monochloramine is that it produces fewer disinfection byproducts compared to chlorine[4]. Some water utilities have switched to monochloramine to meet EPA standards aimed at reducing disinfection byproducts in drinking water[4].
Taste and Odor Benefits
Chloramines are generally less noticeable and less offensive than free chlorine in terms of taste and odor[1]. Water treated with monochloramine often has less of a "chlorine" taste and smell compared to water treated with chlorine alone[4].
While monochloramine offers these benefits, it’s important to note that water treatment facilities often use a combination of disinfection methods. Chlorine is still commonly used for primary disinfection, with monochloramine added for secondary disinfection to maintain water quality throughout the distribution system[4][5].
Chloramine Chemistry & Species
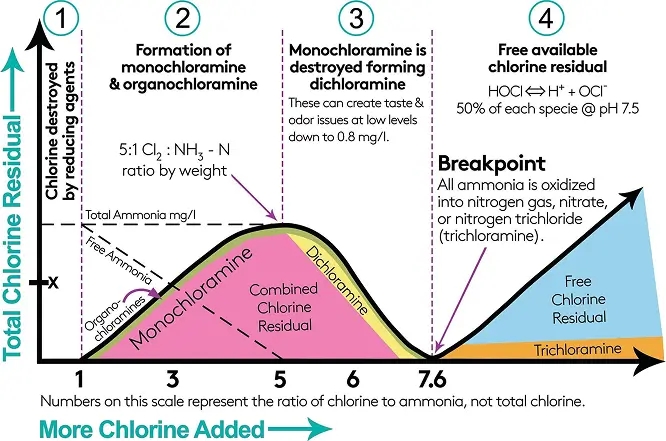
he creation of Chloramines occurs when the weight ratio of Chlorine to Ammonia (Cl2 : NH3) is between 3:1 to 5:1. Anything over 5:1 can lead to the creation of dichloramine. Dichloramine generation will cause unwanted “swimming pool” taste and odor problems and is therefore to be avoided while excess ammonia can lead to nitrification problems.
The ability to measure and control with small amounts to free chlorine in the process will ensure that little to n free ammonia exists and potentially lead to nitrification. This is an important distinction.
The formation of these compounds depends on several factors, including pH, temperature, and the ratio of chlorine to ammonia.
Monochloramine (NH2Cl)
Most common form used in water treatment
Forms when chlorine and ammonia are combined at a specific ratio (usually 4.5:1 to 5:1 chlorine to ammonia)
Provides longer-lasting disinfection than free chlorine
Less likely to form disinfection by-products
Dichloramine (NHCl2) and Trichloramine (NCl3)
Form when the chlorine to ammonia ratio is higher
Can cause taste and odor issues in water
Trichloramine is particularly unstable and typically only forms at low pH levels
Free Chlorine
Refers to chlorine that is available for disinfection (Cl2, HOCl, OCl-)
Reacts with ammonia to form chloramines
More reactive than chloramines but less stable in distribution systems
Ammonia
Reacts with free chlorine to form chloramines
Excess ammonia can lead to nitrification issues in distribution systems
Operational Challenges
Maintaining proper monochloramine levels requires careful management:
Incomplete reaction of precursors can lead to excess free ammonia, potentially causing nitrification in the distribution system. This may require flushing or a “free chlorine” burn to remove biofilm from distribution lines.
The ratio of chlorine and ammonia must be carefully controlled to prevent, excess ammonia, minimize or avoid the formation of dichloramine and trichloramine as they are less effective disinfectants and can cause taste and odor issues.
Nitrification is a challenge due to the potential impact that it can have on distribution system water quality. Nitrification can result in a significant drop in disinfectant residual, which has the potential to result in increased bacteria growth, higher heterotrophic plate count (HPC) and coliform levels, and increased biofilm growth.
As chloramine decays, it releases ammonia. Ammonia is then oxidized to nitrite and then nitrate by bacteria, which leads to biofilm generation and the acceleration of chloramine decay, leading to the release of more ammonia. For example, a 0.40 mg/L nitrite concentration will consume 2.0 mg/L of the chloramine residual. The depletion of chloramine residuals could leave a system vulnerable to bacteriological contamination. A reduction in pH and alkalinity often accompanies nitrification, which increases the potential for the corrosion of lead and copper.
Nitrification can cause a variety of problems and violations within water systems. These steps will help.
Measure monochloramine in the treated water.
Minimize free ammonia in treated water. Verify free ammonia levels periodically with a instrument like the Hach SL1000 and a Free Ammonia Key.
Measurement Difficulties
Accurately measuring monochloramine levels can be challenging:
Traditional measurement methods may overestimate monochloramine concentrations.
Proper testing and monitoring are crucial to maintain effective disinfection while minimizing byproduct formation.
Total Chlorine Measurement Problems
Currently, the most common method used to monitor monochloramine levels in drinking water treatment is by using DPD Total and DPD Free measurements. Monochloramine, a well-known interferent in the DPD Free measurement,
complicates this technique.
The conventional DPD residual control methods do not provide enough information about the break-point chlorination profile. When using a Total Chlorine sensor or DPD measurement, an operator cannot know whether a 3.0 mg/L result is monochloramine, a mixture of chloramines, or free chlorine. If excess ammonia is used, it may lead to nitrification. There is simply no way to know where one is on the breakpoint curve.
The measurement of total chlorine does not allow for the operator to distinguish between the types of chlorine that may be present in the process stream. For this reason, it is important to understand what the measurement can mean and
what condition we desire.
Total chlorine, as the name suggests, measures the total concentration of chlorine species whether they are free chlorine or any of the various chloramine species that exist. In the tables below illustrates this point. It does not matter
whether you have 100% one species or a mix, the total chlorine measurement will be the sum of the concentrations of all the species.
The conditions which we want our sensor to operate in are below (green). Ideally, the monochloramine measurement will be equal to the total chlorine value. If the measured total chlorine value is quite a way from the total value, then the
red conditions are likely.
Chloramination and pH
This is an effective illustration of the three species and what will exist under what ph conditions for a normal water distribution system. Basically after 8pH there will be zero dichloramine and at most maybe about 5% at 7 ph. In practice there will be virtually zero but theoretically it is energetically possible for it to form until about 8 pH in extremely low quantities. at 7.5pH there would probably be a .01 or .02 residual at 1.5ppm The pH level significantly influences the formation of different chloramine species in water. Here’s how pH affects chloramine formation:
pH Impact on Chloramine Formation
At pH 6.5-8.5 (typical drinking water range), monochloramine is the primary species formed
As pH decreases below 7, the formation of dichloramine and trichloramine becomes more favorable
Alkaline conditions (pH > 8) strongly favor monochloramine formation and stability
Chloramination and pH
This is an effective illustration of the three species and what will exist under what ph conditions for a normal water distribution system. Basically after 8pH there will be zero dichloramine and at most maybe about 5% at 7 ph. In practice there will be virtually zero but theoretically it is energetically possible for it to form until about 8 pH in extremely low quantities. at 7.5pH there would probably be a .01 or .02 residual at 1.5ppm The pH level significantly influences the formation of different chloramine species in water. Here’s how pH affects chloramine formation:
pH Impact on Chloramine Formation
At pH 6.5-8.5 (typical drinking water range), monochloramine is the primary species formed
As pH decreases below 7, the formation of dichloramine and trichloramine becomes more favorable
Alkaline conditions (pH > 8) strongly favor monochloramine formation and stability
Measurement Methods
Measurement Methods
Indophenol Method
Free Ammonia
Hach SL1000 Free Chlorine Chem Keys are usually accurate unless there are very high NH4 levels
Online Instruments
Online monochloramine analyzers offer several benefits over traditional methods of monitoring monochloramine levels. They provide continuous monitoring of monochloramine levels, which allows for
Online Monochloramine Analyzers
rapid detection of changes in water quality. Online analyzers also eliminate the need for manual sample collection and analysis, which reduces the risk of human error and increases efficiency.
There are several types of online monochloramine analyzers available in the market. Most use colorimetric methods based on several reagent chemistries Colorimetric analyzers use a reagent that reacts with monochloramine to produce a color change. These analyzers are expensive (over $30K) and require expensive reagents ($4,200 per year) and only update measurement every five minutes. They also require a waste stream of 70,000 gallons per year.
Water treatment plants carefully monitor and control the formation of chloramines
Optimal monochloramine formation occurs at specific chlorine to ammonia ratios
pH control is crucial (typically maintained above 8.0 for monochloramine stability)
Regular testing for free chlorine, total chlorine, and ammonia helps maintain the desired chloramine species
rapid detection of changes in water quality. Online analyzers also eliminate the need for manual sample collection and analysis, which reduces the risk of human error and increases efficiency.
Integrated Free Chlorine Measurement
If a free chlorine distribution “burn” is required, the sensor will automatically display the free chlorine level along with any monochloramine present.
A New Control Method
We propose a new method of monitoring chloramination. In the past, most operators ensured a small free ammonia residual is present in treated water to minimize the potential for dichloramine production. This requires operators to feed an excess of ammonia into the system. Since this new method measures free chlorine without monochloramine interference, a small free chlorine residual of 0.04 to 0.08 ppm will ensure that one is at the top of the breakpoint curve with no excess ammonia. By monitoring both free chlorine and monochloramine, the operator can optimize the process by adjusting the ammonia feed to ensure a very low chlorine residual is present. At pH above 9.0 dichloramine will not form, so a small Free chlorine residual will optimize the process and ensure minimal excess ammonia enters the system.
The sensor is not sensitive to dichloramine and trichloramine, only monochloramine and free chlorine. When paired with the D20 controller, all six parameters are displayed on a single controller.
Since Halogen’s MP6 updates its measurement every two minutes, it can control more precisely than online reagent systems that update every 4.5 minutes. All other amperometric or DPD measurements list monochloramine as an interferent. Hence Halogen MP6 is the only sensor that can be used in this control scheme.
There is a short “warm up” time when the sensor is first started up. This usually takes 10 to 15 minutes.
Free Chlorine
Monochloramine
ORP
pH
Conductivity
Temperature
Halogen MP6 enables a new control method. Instead of ensuring that an excess of free ammonia is present, an operator can maintain a 0.01 to 0.05 ppm Free chlorine residual to ensure that that reaction is optimized near the top of the breakpoint curve. This minimizes excess ammonia in the treated water.
The sensor is based on the MP5 platform, proven in challenging applications:
No reagents
No membrane
No waste stream for water savings of 70,000 gallons per year
Lower initial cost
NSF61 Certified
Lower operating cost
Direct pipe insertion
Self-cleaning
Flow independent
Calibration intervals of 6 months or more

Indophenol Method
Free Ammonia
Hach SL1000 Free Chlorine Chem Keys are usually accurate unless there are very high NH4 levels
Online Instruments
Online monochloramine analyzers offer several benefits over traditional methods of monitoring monochloramine levels. They provide continuous monitoring of monochloramine levels, which allows for
Online Monochloramine Analyzers
rapid detection of changes in water quality. Online analyzers also eliminate the need for manual sample collection and analysis, which reduces the risk of human error and increases efficiency.
There are several types of online monochloramine analyzers available in the market. Most use colorimetric methods based on several reagent chemistries Colorimetric analyzers use a reagent that reacts with monochloramine to produce a color change. These analyzers are expensive (over $30K) and require expensive reagents ($4,200 per year) and only update measurement every five minutes. They also require a waste stream of 70,000 gallons per year.
Water treatment plants carefully monitor and control the formation of chloramines
Optimal monochloramine formation occurs at specific chlorine to ammonia ratios
pH control is crucial (typically maintained above 8.0 for monochloramine stability)
Regular testing for free chlorine, total chlorine, and ammonia helps maintain the desired chloramine species
rapid detection of changes in water quality. Online analyzers also eliminate the need for manual sample collection and analysis, which reduces the risk of human error and increases efficiency.
Integrated Free Chlorine Measurement
If a free chlorine distribution “burn” is required, the sensor will automatically display the free chlorine level along with any monochloramine present.
A New Control Method
We propose a new method of monitoring chloramination. In the past, most operators ensured a small free ammonia residual is present in treated water to minimize the potential for dichloramine production. This requires operators to feed an excess of ammonia into the system. Since this new method measures free chlorine without monochloramine interference, a small free chlorine residual of 0.04 to 0.08 ppm will ensure that one is at the top of the breakpoint curve with no excess ammonia. By monitoring both free chlorine and monochloramine, the operator can optimize the process by adjusting the ammonia feed to ensure a very low chlorine residual is present. At pH above 9.0 dichloramine will not form, so a small Free chlorine residual will optimize the process and ensure minimal excess ammonia enters the system.
The sensor is not sensitive to dichloramine and trichloramine, only monochloramine and free chlorine. When paired with the D20 controller, all six parameters are displayed on a single controller.
Since Halogen’s MP6 updates its measurement every two minutes, it can control more precisely than online reagent systems that update every 4.5 minutes. All other amperometric or DPD measurements list monochloramine as an interferent. Hence Halogen MP6 is the only sensor that can be used in this control scheme.
There is a short “warm up” time when the sensor is first started up. This usually takes 10 to 15 minutes.
Free Chlorine
Monochloramine
ORP
pH
Conductivity
Temperature
Halogen MP6 enables a new control method. Instead of ensuring that an excess of free ammonia is present, an operator can maintain a 0.01 to 0.05 ppm Free chlorine residual to ensure that that reaction is optimized near the top of the breakpoint curve. This minimizes excess ammonia in the treated water.
The sensor is based on the MP5 platform, proven in challenging applications:
No reagents
No membrane
No waste stream for water savings of 70,000 gallons per year
Lower initial cost
NSF61 Certified
Lower operating cost
Direct pipe insertion
Self-cleaning
Flow independent
Calibration intervals of 6 months or more


Until now, operators have had to use very expensive, complex systems that require frequent reagent replacements and are difficult to install. Now it is possible to use a small In-pipe NSF61 Certified sensor that uses a proven hardware platform that provides valuable insight into the chloramination process, even in the distribution system.
Conclusion
